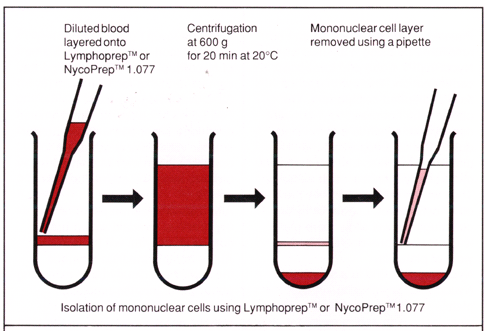
Differential WBC Subpopulation Isolation
Again, as illustrated in the figure at the right, different WBC subpopulations can be harvested from different buoyant "layers" following centrifugation through a density gradient. Here for example, a polysaccharide solution of density = 1.077 g/ml (e.g., Ficoll-400) is shown to separate other blood cells from peripheral blood mononuclear cells (