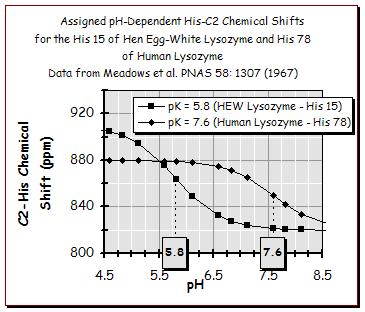
| HEW lysozyme pK (His 15) = 5.8 | Human Lysozyme pK (His 78) = 7.6 |
|---|
Lysozyme is a glycosidase and enzymes isolated from different vertebrate species are found to be highly homologous in terms of their amino acid sequences and their catalytic mechanisms and chemistry. For example, it is found that the primary amino acid sequences of human lysozyme and chicken lysozyme, or so-called hen eqq-white lysozyme (HEWL), differ by only one residue in length and have over 30% sequence identity with 3-D folded protein structures that produce virtually identical enzyme active sites.
The amino acid sequences of both enyzmes are also found to include only a single histidine residue but these HIS residues are located in nonhomologous positions in the two amino acid sequences of these enzymes and in different structural locations in the folded 3-D enzymes as shown in the JSmol images on the right.
In one study, differences in the local microenvironments surrounding the His imidazole sidechain conjugated ring of this residue was specifically
View Excel spreadsheet
Meadows et al. PNAS 58: 1307 (1967)