VI. Binding to MHC/Peptide Ligands
Go back
 Click here to load TCR-MHC
structure
Click here to load TCR-MHC
structureIn the structure of the 2C TCR complexed with
its class I MHC(H-2Kb)-peptide(dEV8) ligand, several
hallmarks of T cell antigen recognition are evident. The basis
for MHC restriction is elucidated, as the TCR's primary contact
is made with the alpha helices of the H-2Kb molecule,
which shield the dEV8 peptide in the binding groove. The
relatively high incidence of TCR alloreactivity and the ability
of the TCR to bind multiple ligands (a prerequisite for thymic
selection) are similarly explained by the structural plasticity
and unoptimized shape complementarity of the binding site.
Finally, the orientation of the six CDR loops reveals the TCR's
ability to "scan" several MHC molecules for optimal
interaction with bound peptide.
Each CDR loop of the TCR performs a specific
function in binding the H-2Kb-dEV8 ligand.
CDR2α and
2β lie directly above
the α2
and α1
helices of H-2Kb, respectively, and interact
exclusively with the MHC framework. The two CDR1 loops are
positioned to bind both peptide and MHC residues, with CDR1α docking above the
amino-ternimus of the bound peptide and CDR1β docking above the
carboxy-terminus. The two CDR3 loops extend into the
peptide-binding groove and interact directly with the antigenic
peptide, making minimal contact with the MHC framework. The fine
specificity of antigen binding is thus determined primarily by CDR3α
and CDR3β.
While CDR3α and
β finely discriminate among bound peptides,
the remaining CDR loops provide a general binding orientation to
the MHC ligand. A standard docking engagement allows the TCR to
superficially interact with several different MHC-peptide
complexes, inducing the activation signal only upon binding a
strongly antigenic ligand. This interaction of CDRs 1 and 2 with H-2Kb
predominantly occurs at
conserved residues on the MHC helices, and forms the basis for
the class I or class II restriction of the TCR. Ser27α(CDR1) and
Ser51α(CDR2)
make critical hydrogen bonds with the conserved H-2Kb
residues Glu58 and Glu166 to help define the binding orientation
and MHC restriction of the 2C TCR.
|
CDRs 1 and 2 binding to H-2Kb
|
When compared with the TCR's strong interaction
with H-2Kb, the complementarity of CDR3α and
b with the dEV8
peptide is surprisingly weak. (dEV8 is a self peptide derived
from the murine mitochondrial respiratory protein complex and has
the sequence EQYKFYSV.) The peptide's most prominent structural
features are two fully extended residues at Lys4 and
Tyr6 which
seem ideally positioned to engage the TCR. The lack of
complementary residues on the CDR3 loops, however, prevents this
interaction. CDR3α forms only three weak polar associations with the
charged nitrogen atom of Lys4, all of which are derived from main
chain carbonyl groups. CDR3β accomplishes only one hydrophobic contact with
Tyr6,
and the prominent cleft between the two CDRs remains empty. The
large proportion of empty space within the binding pocket
contributes to the low affinity of the complex, and suggests that
the dEV8 peptide may act as a generic antigen for several TCRs
during thymic development.
The weak association between the 2C TCR and the
self MHC-self peptide complex of H-2Kb-dEV8
acts to positively select thymocytes expressing the 2C TCR. The
fact that this same interaction cannot elicit a strong
activation signal in the periphery highlights the dual nature of
intracellular signaling through the TCR. A much stronger ligand
for the 2C TCR is the H-2Kb complex with the synthetic
peptide SIYRYYGL, which causes negative selection in the thymus
as well as robust activation in the periphery. Presumably, the
substitution of upward-facing residues in the center of the
peptide allows for better shape complementarity with the CDR3
loops, resulting in activation through the TCR complex.
In a similar manner, alteration of
the MHC ligand can lead to increased signaling through the TCR.
When the dEV8 peptide is bound to H-2Kbm3(a
2 amino
acid mutant of H-2Kb), thymocytes bearing the 2C TCR
are negatively selected while mature T cells in the periphery are
induced to proliferate. The structurally important mutation in
this case occurs at residue 77 of H-2Kb
(D77S), where
the loss of an aspartate residue disrupts a critical hydrogen
bond with a main chain carbonyl of the peptide. This results in
altered presentation to the receptor and greatly increased
binding affinity with the 2C TCR.
|
H-2Kb
(Asp77) interaction with dEV8 |
The examples above reveal how
changes in the structure of either the MHC or peptide ligand can
increase the activation signal delivered through the TCR complex.
It is not clear, however, how alterations in the binding affinity
of MHC-peptide ligands are translated into intracellular
activation signals. Because a crystallographic structure of the
TCR bound to a strongly agonistic ligand has not yet been solved,
predictions must be made from the present complex of 2C-H-2Kb-dEV8.
Firstly, the complex exists as a monomer, which contradicts both
the observation that class II MHC molecules crystallize as dimers
and the prediction that TCR signaling occurs through
multimerization of the TCR-MHC-peptide complex. Secondly, the
four domains of the 2C TCR have maintained the same orientations
and internal structure as found in the unliganded receptor,
including the energetically-unstable Ca domain. This supports the
model of a structurally rigid TCR, and challenges the notion of a
conformational change to induce intracellular activation. Minor
alterations in the structure of the TCR variable domains,
however, do occur upon antigen binding, perhaps indicating
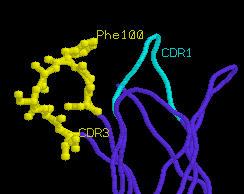
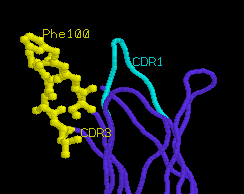
greater conformational rearrangement upon true activation. Most
notably, CDR1α is angled away from the peptide binding groove, and
CDR3α
is displaced to accommodate the extended side chain of peptide
residue Lys4. The movement of CDR3α is quite dramatic (6
angstroms), and is accompanied by internal rotation of extended
amino acid side chains such as Phe100α.
unliganded TCR
α
chain
|
liganded TCR
α
chain
|
 |
 |
Until more structures of liganded T cell receptors are
available, the exact nature of TCR antigen binding and
intracellular activation will remain a mystery. From the 2C
structure, however, it appears that the TCR is designed to
recognize antigen-presenting MHC molecules from a pre-determined
orientation. This may allow the TCR to effectively
"scan" several antigens until a precise complementarity
of fit is obtained with the bound peptide. Higher affinity
binding may result in either a conformational change or the
aggregation of the receptor, or perhaps subtle conformational
changes allow the TCR to form multimers when bound to its
MHC-peptide ligands. In any case, the T cell receptor is
remarkably adept at recognizing foreign peptides in the context
of self MHC molecules, while the TCR complex as a whole initiates
a variable level of intracellular activation for thymic selection
or clonal expansion.

