Complement (C') System
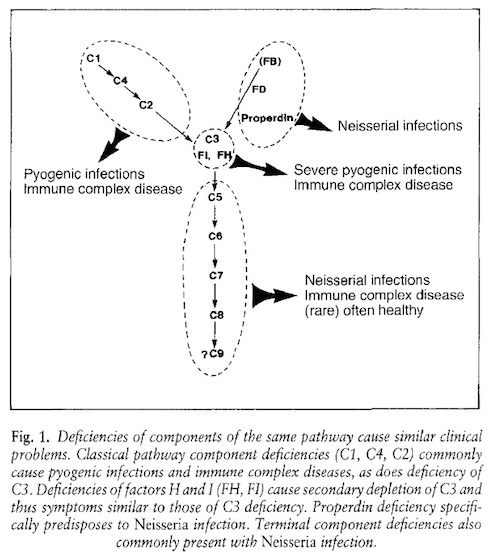
Immune Protection by C' and the Consequences of C' Deficiencies Several C' activation steps produce inflammatory factors that promote immune responses against many types of bacteria and even some viruses packaged inside plasma membranes. Not surprisingly, a deficiency - either inherited or acquired - in any one of the C' components is often accompanied by an increased susceptibility to infectious disease. In some cases, deficiencies, particularly those linked to the classical C' pathway, are typically associated with increased susceptibility to infections by extracellular pyogenic (pus forming) microbes. These individuals are also more likely to suffer from immune complex disease (ICD), particularly those with C1q deficiencies (1). Individuals with deficiencies in alternate C' pathway components are particularly susceptible to certain "high-grade " pathogens - for example, certain Pneumococcal, Staphylococcal, Neisserial, or Haemophilus microbes that cause serious systemic illnesses like meningitis or gonorrhea.
B. Paul Morgan and Mark J. Walport. Complement deficiency and disease.Immunology Today 12:301-6 (1991)
C' Activation Pathways
The C' system and its receptors are defined by nearly two dozen proteins, most of which exist in significant concentrations in serum. Under normal conditions, C' proteins exist as inactive enzymes that generally become activated (as serine proteases) in a progressive, cascading fashion when the system is activated, usually in response to certain types of immunological triggering events. For example, antibody/antigen complexes trigger the classical pathway of C' activation (highlighted inside the blue rectangle at the left). Conversely, the alternate pathway of C' activation (highlighted inside the green rectangle at the
left) is triggered by certain pathogen-derived molecules when present. This pathway, however, is also spontaneously activated when certain C' regulatory molecules are absent or defective. In any case, both pathways converge with respect to the downstream C' component activation steps (highlighted inside the black rectangle at the left). The potential C' activity in isolated human samples can be measured quantitatively for both pathways by a relatively simple C' hemolytic assay.
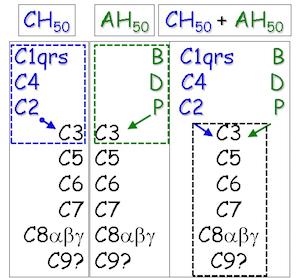
Identifying C' Component Deficiencies
- If CH50, but not AH50, is abnormally low then it is likely that C1, C2, and/or C4 is/are nonfunctional, depleted, or present in suboptimal concentration(s).
- If AH50, but not CH50, is abnormally low then it is likely that FB, FD, and/or P is/are nonfunctional, depleted, or present in suboptimal concentration(s).
- If both CH50 and
AH50 are abnormally low, then it is likely that C3, C5, C6, C7, C8, and/or C9 is/are nonfunctional, depleted, or present in suboptimal concentration(s).
Some Possible Causes for C' Component Deficiencies
- Human congenital defects have been described for most of the complement components, regulatory proteins, and receptors.
- Excessive consumption of one or more complement component can result from the following:
- a defective C' regulatory factor (e.g., Factor I, etc.).
- pathogen alteration of the activity of a C' component.
- an unchecked pathogen infection.
References
- G. J. Arason, G. H. Jorgensen, and B. R. Ludviksson. Primary immunodeficiency and autoimmunity: Lessons from human diseases. Scand. J. Immunol. 71:317-328 (2010).
- G. J. Arlaud, C. Gaboriaud, N. M. Thielens, and V. Rossi. Structural biology of C1. Biochem Soc Trans. 30:1001-6 (2002).
- C. L. Villiers, G. J. Arlaud, and M. G. Colomb. Domain structure and associated functions of subcomponents Clr and Cls of the first component of human complement. Proc. Natl. Acad. Sci. USA 82:4477-81 (1985).
- K. Yonemasu, T. Sasaki, and H. Shinkai. Purification and characterization of subcomponent Clq of the first component of bovine complement. J. Biochem. 88:1545-54 (1980).
- R. C. Strunk, A. S. Whitehead, and F. S. Cole. Pretranslational regulation of the synthesis of the third component of complement in human mononuclear phagocytes by the lipid A portion of lipopolysaccharide.
J. Clin. Invest. 76:985-90 (1985).
- G. Goldberger and H. R. Colten. Precursor complement protein (pro-C4) is converted in vitro to native C4 by plasmin. Nature 286: 514-6 (1980).
- R. A. Wetsel and W. P. Kolb. Complement-independent activation of the fifth component (C5) of human complement: Limited trypsin digestion resulting in the expression of biological activity. J. Immunol. 128:2209-16 (1982))
- B. Scheurer, C. Rittner, P. M. Schneider. Expression of the human complement C8 subunits is independently regulated by interleukin 1β, interleukin 6, and interferon γ. Immunopharmacol. 3:167-75 (1997).