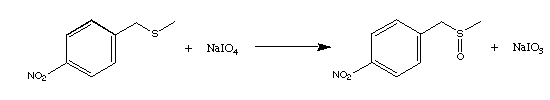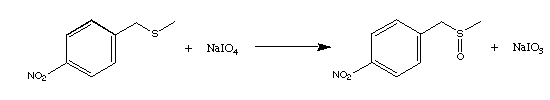By exploiting the highly
specific antigen binding properties of antibodies, experimental strategies have been
devised to produce antibodies that catalyze chemical reactions. These catalytic
antibodies, or abzymes, are selected from monoclonal antibodies generated by
immunizing mice with haptens that mimic the transition states of enzyme-catalyzed
reactions. For example, the 28B4 abzyme catalyzes periodate
oxidation of p-nitrotoluene-methyl sulfide to sulfoxide, as shown below, where electrons from the sulfur atom are transferred to
the more electronegative oxygen atom.
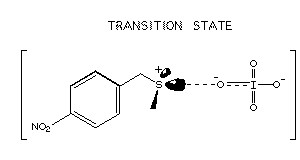
The rate of this reaction
is promoted by enzyme catalysts that stabilize the transition
state of this reaction, thereby decreasing the activation
energy and allowing for more rapid conversion of substrate to product. In this case, the
transition state is thought to involve a transient positive charge on the sulfur atom and
a double-negative charge on the periodate ion as shown below on the left.
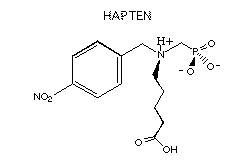
In order to generate
abzymes complementary in structure to this transition state, mice were immunized with an aminophosphonic acid hapten, as shown above
at the right. Obviously, its structure mirrors the structure and electrostatic properties
of the sulfoxide transition state. Of the hapten-binding monoclonal antibodies produced
with this hapten, many were found to catalyze sulfide oxidation but with a wide range of
binding affinities and catalytic efficiencies. In particular, abzyme 28B4 binds hapten
with high affinity (Kd = 52 nM) and exhibits a correspondingly high degree of
catalytic efficiency (k3/KM = 190,000 M-1s-1).
Elucidation of the molecular
structure of abzyme 28B4 bound to the hapten reveals much about the nature of its
catalytic action. Highly specific structural and electrostatic interactions create a
remarkable degree of structural complementarity between the antigen-binding site and the
sulfoxide transition state analog as illustrated in the following series of
three-dimensional views of the antibody-hapten complex.
Reset
|
Zoom
out
|
Zoom
in
|
Spacefill
hapten
|
Spacefill
active site
|
Hapten
only
|
Active
site only
|
|
| 1kel.pdb |
reload
page |
 |
|
|
Rotate
around active site complex. |
|
|
Label
charged or partially charged centers. |
|
|
Labels off. |
|
|
|
For best
results, push the buttons in sequence. |
|
|
1. |
Zoom into the
antigen-binding site with bound hapten. Initially, the hapten
is visible in the 28B4 Fab fragment bound to the antigen-binding site
locted between the H chain and L chain. |
|
|
2. |
Zoom in to view the
structural complementarity between antibody and hapten. |
|
|
3. |
Highlight
ionic interactions and hydrogen bonding between sidechain
atoms of Arg52, Lys56, & Tyr33 (all in the H chain) and
the hapten's anionic phosphate. |
|
|
4. |
Highlight
hydrophobic and aromatic interactions between several
antibody sidechains and the hapten's aromatic group. |
|
|
5. |
Highlight
p-electron stabilization by the aromatic group of Tyr37 (L
chain) and the tetrahedral,
cationic nitrogen atom of the hapten. |
|
|
6. |
Highlight
hydrogen bonding between the sidechain of Asp35 (H chain) and the hapten's electronegative p-nitro (-NO2) group. |
|
|
Analysis of abzyme 28B4
without bound hapten reveals a slightly altered hapten binding site conformation,
suggesting that the induced-fit model of enzyme-substrate binding is a feature of the
abzyme's catalytic mechanism. Also, sequence analyses of other antibodies that bind this
hapten hint at a mechanism for enzyme evolution. Of particular interest is asparagine 35 of
the 28B4 heavy chain which forms a key hydrogen bond with the p-nitro group of
the substrate, dramatically increasing the specificity the 28B4 abzyme compared to other
hapten-specific antibodies that have a different amino acid residue at this position. The
germline sequences of the heavy chain gene segments indicate that this particular
asparagine residue arose as the result of somatic mutation of the heavy chain gene during
affinity maturation of hapten-specific B lymphocytes. Thus, one can begin to comprehend
the evolution of intricately specific enzymes from less-active or inactive protein
precursors. The study of catalytic antibodies as a whole has vastly increased current
understanding of the mechanisms of enzyme catalysis and represents another step forward in
the attempts to create artificially engineered biological enzymes.
Based on the experimental data of
L.C.Hsieh-Wilson, P.G.Schultz, and R.C.Stevens. Proc. Natl. Acad. Sci. USA, 93:5363-5367
(1996).