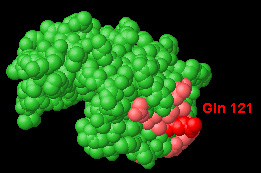
 EPITOPE of HEL Hen Egg-white Lysozyme |
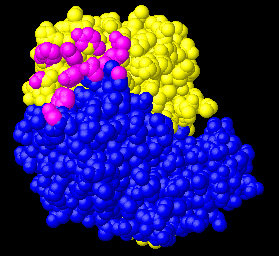 PARATOPE of Anti-HEL Fab (L:H) |
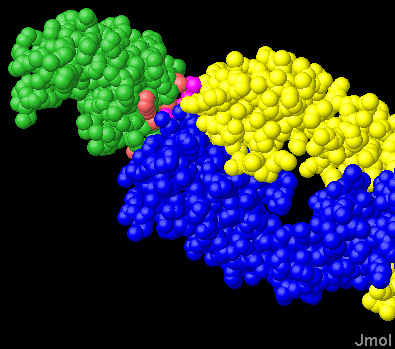 Complex between HEL and the papain fragment - Fab (L:H chain fragment) - of an anti-HEL antibody. |
|
 EPITOPE of HEL Hen Egg-white Lysozyme |
 PARATOPE of Anti-HEL Fab (L:H) |
 Complex between HEL and the papain fragment - Fab (L:H chain fragment) - of an anti-HEL antibody. |
|