Show me



r / [L] vs. r
r = n * Ya = the average number of ligand molecules bound to a receptor with r ligand binding sites
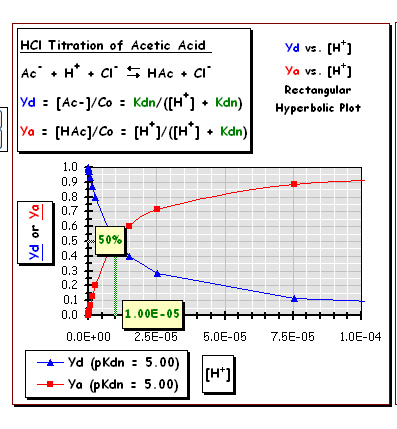

Yd vs. [H+] (log scale)
Base Titration Plot

pH vs. Yd

log (Ya / Yd ) vs. log [L]

Yd vs. pH
Vo / Vmax
vs. [S]
Vmax = the initial reaction velocity
Vo/ Vmax is mathematically equivalent to Ya
Vmax = the initial reaction velocity
Vo/ Vmax is mathematically equivalent to Ya
