-
Move the mouse
pointer over the image to switch between the oxyHbA
and deoxyHbA conformations of adult
hemoglobin A their respective oxygen saturation levels in arterial (red)
or venous (blue) blood.
-
Oxygen binding by Hb and its release are accompanied by noticeable conformational changes
in the structure of Hb.
-
As Hb is transported in red blood cells
between the lungs and the capillaries, continuously oscillates between
two (and probably more) conformational extremes.
-
In the "deoxyHb" conformation,
which predominates in venous blood, hemoglobin's oxygen affinity is
relatively low.
-
In the "oxyHb" conformation,
which predominates in arterial blood, hemoglobin's oxygen affinity is
relatively high.
-
In effect, Hb
is like an "oxygen sponge" when it oscillates
between these conformation extremes.
|
Roll mouse over theimage below to switch between deoxyHb & oxyHb structure and function.
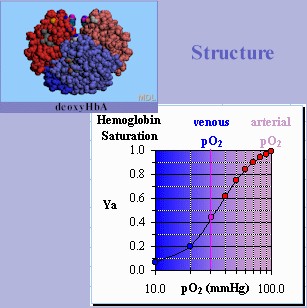 |
-
As illustrated by the chart, the amount of oxygen bound to
- Hb, i.e., the saturation fraction, Ya, is measured a function of the pressure of oxygen gas in the immediate environment, pO2.
- In
arterial blood leaving the lungs, Hb has up to four oxygen
molecules bound being nearly 100% saturated in this environment.
A high percentage of oxygen-saturated oxyHb
molecules gives arterial blood its slightly pinkish coloration.
-
In the
capillaries or venous blood, Hb
releases some its bound oxygen molecules, becoming desaturated of
oxygen in this environment. A high percentage of
oxygen-depleted deoxyHb
molecules gives venous blood it is slightly bluish coloration.
|

