Click to change pH.
Click to start animation.
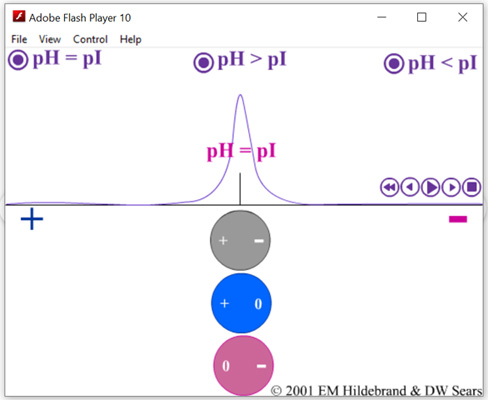

Example: The Isoelectric pH, or pI, of Glycine equals 6.1. |
||||
|
||||
4 Possible Microstates |
Equilibrium
Microstate Fractions at pH = 6.1 |
|||
| (+ -): NH3+-CH2-COO- | At pH = 6.1, fraction = Ya(a-NH3+) * Yd(a-COO-) = 99.96% | |||
| (+ 0): NH3+-CH2-COOH | At pH = 6.1, fraction = Ya(a-NH3+) * Ya(a-COOH) = 0.02% | |||
| (0 -): NH2-CH2-COO- | At pH = 6.1, fraction = Yd(a-NH2) * Yd(a-COO-) = 0.02% | |||
| (0 0): NH2-CH2-COOH | At pH = 6.1, fraction = Yd(a-NH2) * Ya(a-COOH) = 0.000004% | |||
|
||||